Familiarize staff While using the regulatory requirements and suggestions governing Microbial Limit Testing. This makes sure that testing procedures align with industry prerequisites and keep compliance with pertinent authorities.
While in the pharmaceutical, foods, and beauty industries, guaranteeing the microbial quality of Uncooked resources and finished products is crucial for preserving solution safety and compliance with regulatory expectations. The Quality Control (QC) Department plays a pivotal role in conducting Microbial Limit Tests, employing stringent procedures to copyright solution quality.
However, the complexity and cost associated with this technique can be obstacles for a few institutions, limiting its prevalent use.
Employ immediate corrective steps to handle the determined root result in. This will contain retesting, segregating afflicted batches, or adjusting production processes to stop recurrence.
"Water high-quality is a cornerstone of general public health and fitness, building the enforcement of microbial limits vital to condition prevention and Neighborhood perfectly-currently being."
Deviations pose a chance to merchandise quality, likely resulting in non-compliance with regulatory expectations. Addressing deviations promptly is critical to forestall compromised more info merchandise security and protect the name from the Group.
Deliver training on conducting extensive root bring about analyses to recognize the source of deviations. Encourage a systematic method of investigating and resolving concerns.
To explain procedure for examining complete practical rely, complete fungal rely and Pathogens in finished products.
If there is not any progress of this sort of kind of colonies, or maybe the identification tests are destructive it suggests absence of Salmonella aboney and the sample passes the test.
This involves guaranteeing that staff members members are sufficiently educated on testing procedures, aseptic approaches, and any updates to protocols. Competency assessments are executed to confirm the proficiency of testing staff.
This doc discusses sterility testing solutions In keeping with many pharmacopoeias. It provides particulars on membrane filtration and immediate inoculation procedures for testing sterility of pharmaceutical products like injections and ophthalmic preparations.
A multi-faceted here threat evaluation tactic may incorporate shopper behavioral studies. Being familiar with how people communicate with products aids in assessing their basic safety actions.
Food items security is another significant spot influenced by microbial limits. Microbial contamination can occur at any position from farm to desk. Rules governing meals protection goal to attenuate pitfalls connected with harmful microorganisms in food products.
This short article outlines the precise records that needs to be meticulously managed in the course of Microbial Limit Testing of Uncooked supplies and completed products.
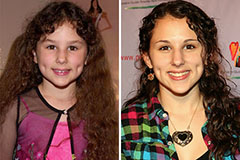 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now!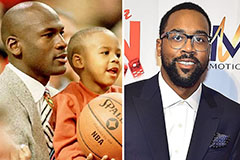 Marcus Jordan Then & Now!
Marcus Jordan Then & Now! Richard Thomas Then & Now!
Richard Thomas Then & Now! Brooke Shields Then & Now!
Brooke Shields Then & Now! Barbara Eden Then & Now!
Barbara Eden Then & Now!